Standard Electrode Potential Niobium . Web the reference state for amalgams is an infinitely dilute solution of the element in hg. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The temperature coefficient, de /dt allows us to calculate. All other species are aqueous. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Reduction reactions in acidic solution are written. Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. Web solids, gases, and liquids are identified;
from pandai.me
Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. Web the reference state for amalgams is an infinitely dilute solution of the element in hg. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The temperature coefficient, de /dt allows us to calculate. Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Web solids, gases, and liquids are identified; Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Reduction reactions in acidic solution are written. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Web although niobium [and tantalum] has two electrons in the outermost shell and it.
Standard Electrode Potential
Standard Electrode Potential Niobium Web although niobium [and tantalum] has two electrons in the outermost shell and it. The temperature coefficient, de /dt allows us to calculate. Web the reference state for amalgams is an infinitely dilute solution of the element in hg. All other species are aqueous. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Reduction reactions in acidic solution are written. Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Web solids, gases, and liquids are identified; Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions.
From www.researchgate.net
Precipitation efficiency. The figure reports precipitation efficiency Standard Electrode Potential Niobium Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. Web solids, gases, and liquids are identified; Web although niobium [and tantalum] has two electrons in the. Standard Electrode Potential Niobium.
From thechemistrynotes.com
Electrode Potential Types, Importance, and Applications Standard Electrode Potential Niobium Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web although niobium [and tantalum] has two electrons in the outermost shell and it. The temperature coefficient,. Standard Electrode Potential Niobium.
From www.researchgate.net
The potentialpH diagrams for ruthenium compounds in the system RuH 2 Standard Electrode Potential Niobium Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. All other species. Standard Electrode Potential Niobium.
From www.just-auto.com
Who are the leading innovators in niobium electrode batteries for the Standard Electrode Potential Niobium The temperature coefficient, de /dt allows us to calculate. Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. All other species are aqueous. Web in electrochemistry,. Standard Electrode Potential Niobium.
From www.chegg.com
The standard potential for the following galvanic Standard Electrode Potential Niobium Web the reference state for amalgams is an infinitely dilute solution of the element in hg. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or. Standard Electrode Potential Niobium.
From pubs.acs.org
Accurate Potentials of Hg/HgO Electrodes Practical Parameters for Standard Electrode Potential Niobium Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The temperature coefficient, de /dt allows us to calculate. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or. Standard Electrode Potential Niobium.
From scienceinfo.com
Measuring the Standard Electrode Potential (Eꝋ) Standard Electrode Potential Niobium Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Web solids, gases, and liquids are identified; Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web the reference state for amalgams is an infinitely dilute solution of the element in hg. All other species are aqueous. Web. Standard Electrode Potential Niobium.
From www.reddit.com
2019 Nobel Prize in Chemistry Discussion Thread r/science Standard Electrode Potential Niobium Web the reference state for amalgams is an infinitely dilute solution of the element in hg. Reduction reactions in acidic solution are written. The temperature coefficient, de /dt allows us to calculate. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Web 183 rows the table is. Standard Electrode Potential Niobium.
From askfilo.com
How would you determine the standard electrode potential of the system Mg.. Standard Electrode Potential Niobium Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Web the potential of the standard hydrogen electrode (she) is defined as 0 v. Standard Electrode Potential Niobium.
From askfilo.com
Problem 11.1Standard electrode potential values, E⊖ for Al3+/Al is −1.66 Standard Electrode Potential Niobium All other species are aqueous. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Web solids, gases, and liquids are identified; The temperature. Standard Electrode Potential Niobium.
From www.researchgate.net
(Color online) Sketch of the side NS junction between a niobium Standard Electrode Potential Niobium Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Web solids, gases, and liquids are identified; Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web the reference state for amalgams is an infinitely dilute solution of the element in hg. Web. Standard Electrode Potential Niobium.
From askfilo.com
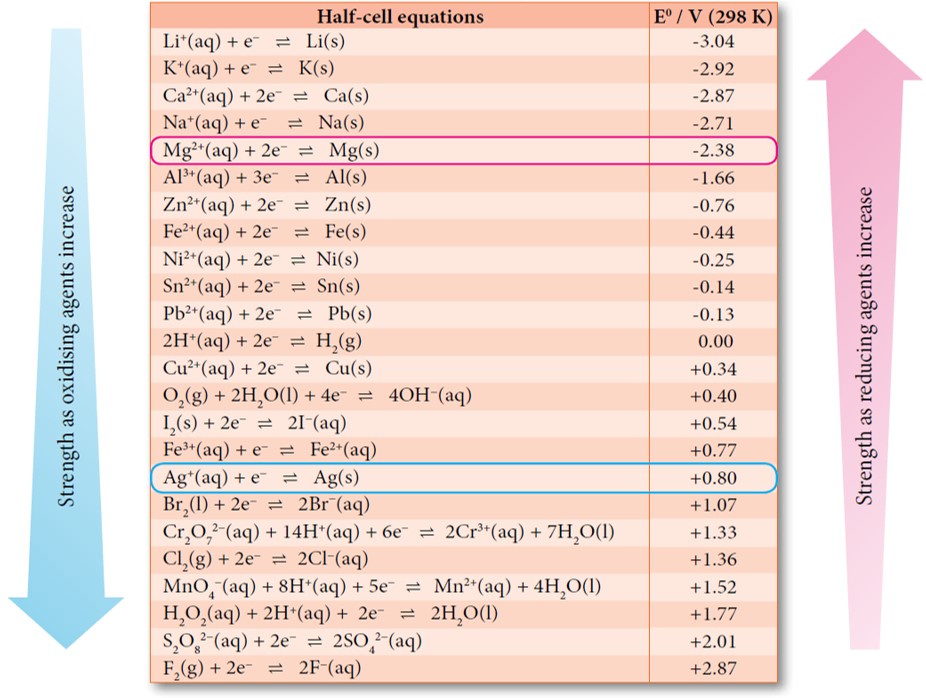
Table The Standard Electrode Potentials at 298 K Redox Reactions 57 Li+.. Standard Electrode Potential Niobium Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The temperature coefficient, de /dt allows us to calculate. Web the potential of the standard hydrogen electrode. Standard Electrode Potential Niobium.
From chemistry.stackexchange.com
everyday chemistry Accelerated Oxidation Of Iron When Coating Breaks Standard Electrode Potential Niobium All other species are aqueous. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Web solids, gases, and liquids are identified; Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web the potential of the standard hydrogen electrode (she) is defined as 0. Standard Electrode Potential Niobium.
From exomimdmo.blob.core.windows.net
Representation Of Standard Hydrogen Electrode at Rona Loomis blog Standard Electrode Potential Niobium Reduction reactions in acidic solution are written. Web the potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. All other species are aqueous. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web in electrochemistry, standard electrode potential ,. Standard Electrode Potential Niobium.
From pubs.acs.org
Accurate Potentials of Hg/HgO Electrodes Practical Parameters for Standard Electrode Potential Niobium Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Reduction reactions in acidic solution are written. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top. Standard Electrode Potential Niobium.
From evulpo.com
evulpo Electrode potentials Standard Electrode Potential Niobium Web in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. Web although niobium [and tantalum] has two electrons in the outermost shell and it. Web the reference state for amalgams is an infinitely dilute solution of the element in hg. Web 183 rows the table is ordered such that. Standard Electrode Potential Niobium.
From www.studocu.com
Chemistry (Term2) 2022 Set 18 SECTION A Given that the standard Standard Electrode Potential Niobium Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. The temperature coefficient, de /dt allows us to calculate. All other species are aqueous. Web 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.. Standard Electrode Potential Niobium.
From askfilo.com
Consider the following standard electrode potential values Fe3+(aq)+e−→.. Standard Electrode Potential Niobium Web the cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode potentials. All other species are aqueous. Web 183 rows the table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at. Web solids, gases, and liquids are identified; The temperature coefficient, de /dt. Standard Electrode Potential Niobium.